What Metals React With Aluminum . aluminium metal reacts vigorously with all the halogens to form aluminium halides. aluminium is unusual, because it is a reactive metal that does not react with water. More reactive metals displace less reactive metals from. So, it reacts with chlorine, cl 2, bromine, i 2,. aluminium reacts with oxygen, forming a protective layer of alumnium(iii) oxide that prevents further reaction with oxygen. Its surface forms a protective layer. dive into the world of aluminium chemistry and its unique reactivity. 9 rows the reactivity series ranks metals by how readily they react. long tabular form of the reactivity series. Group 1 contains reactive metals, group 7. The reactivities of metals are tabulated below (in the descending order) along with their.
from www.teachoo.com
9 rows the reactivity series ranks metals by how readily they react. So, it reacts with chlorine, cl 2, bromine, i 2,. aluminium metal reacts vigorously with all the halogens to form aluminium halides. Its surface forms a protective layer. dive into the world of aluminium chemistry and its unique reactivity. More reactive metals displace less reactive metals from. The reactivities of metals are tabulated below (in the descending order) along with their. aluminium reacts with oxygen, forming a protective layer of alumnium(iii) oxide that prevents further reaction with oxygen. long tabular form of the reactivity series. aluminium is unusual, because it is a reactive metal that does not react with water.
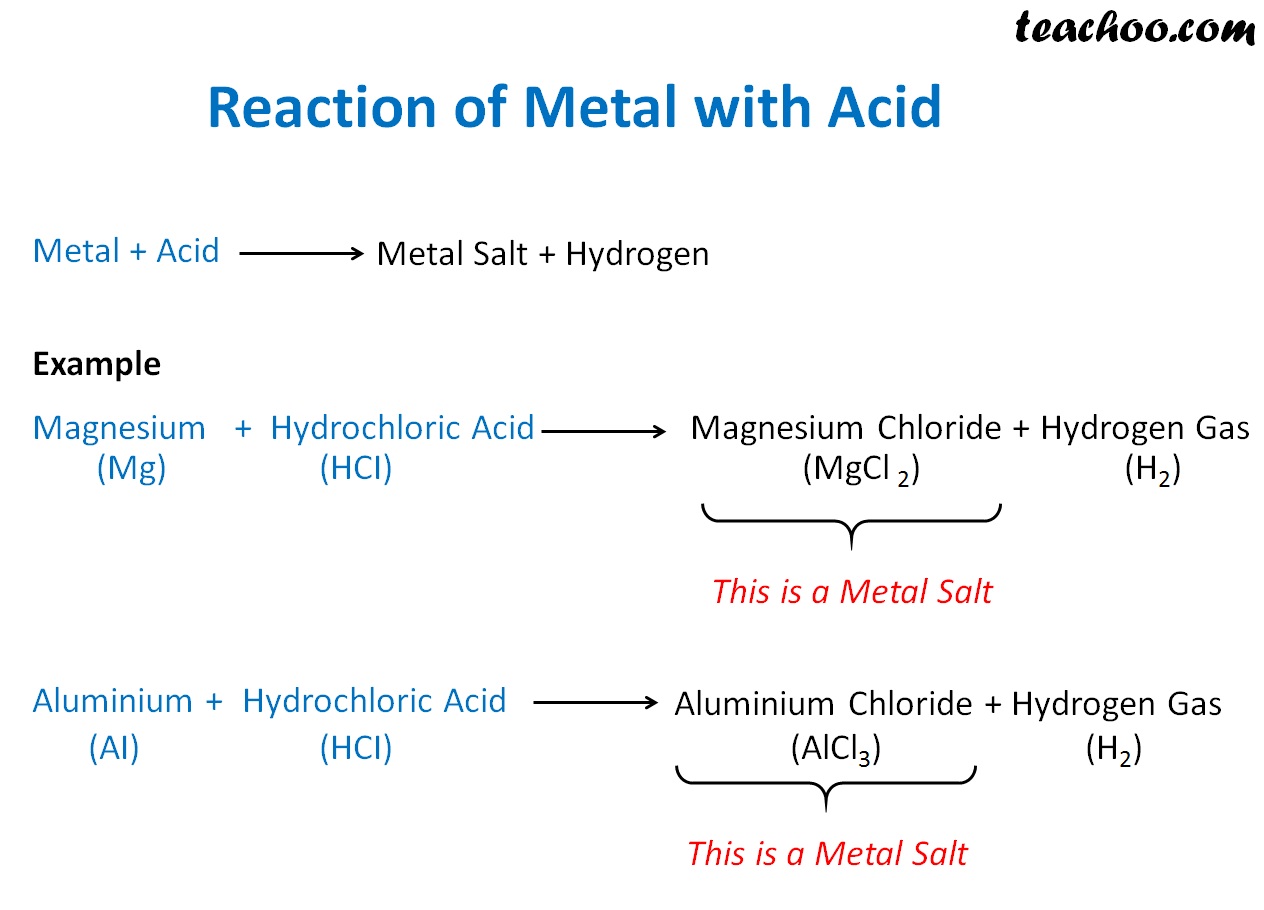
Reaction of Metals and NonMetals with Acids Teachoo Concepts
What Metals React With Aluminum aluminium reacts with oxygen, forming a protective layer of alumnium(iii) oxide that prevents further reaction with oxygen. Group 1 contains reactive metals, group 7. The reactivities of metals are tabulated below (in the descending order) along with their. aluminium reacts with oxygen, forming a protective layer of alumnium(iii) oxide that prevents further reaction with oxygen. long tabular form of the reactivity series. aluminium is unusual, because it is a reactive metal that does not react with water. dive into the world of aluminium chemistry and its unique reactivity. aluminium metal reacts vigorously with all the halogens to form aluminium halides. 9 rows the reactivity series ranks metals by how readily they react. So, it reacts with chlorine, cl 2, bromine, i 2,. More reactive metals displace less reactive metals from. Its surface forms a protective layer.
From www.youtube.com
Reaction of Water with Aluminium Chloride YouTube What Metals React With Aluminum aluminium metal reacts vigorously with all the halogens to form aluminium halides. Its surface forms a protective layer. dive into the world of aluminium chemistry and its unique reactivity. The reactivities of metals are tabulated below (in the descending order) along with their. long tabular form of the reactivity series. 9 rows the reactivity series ranks. What Metals React With Aluminum.
From mrcartlidgesaigon.edublogs.org
C10 Metals Science) Mr Cartlidge's second Science Blog What Metals React With Aluminum aluminium reacts with oxygen, forming a protective layer of alumnium(iii) oxide that prevents further reaction with oxygen. aluminium is unusual, because it is a reactive metal that does not react with water. Its surface forms a protective layer. The reactivities of metals are tabulated below (in the descending order) along with their. 9 rows the reactivity series. What Metals React With Aluminum.
From www.slideserve.com
PPT CHAPTER 3 METALS AND NON METALS PowerPoint Presentation ID598959 What Metals React With Aluminum So, it reacts with chlorine, cl 2, bromine, i 2,. dive into the world of aluminium chemistry and its unique reactivity. Its surface forms a protective layer. long tabular form of the reactivity series. aluminium reacts with oxygen, forming a protective layer of alumnium(iii) oxide that prevents further reaction with oxygen. Group 1 contains reactive metals, group. What Metals React With Aluminum.
From revisechemistry.uk
Obtaining and Using Metals Edexcel T4 revisechemistry.uk What Metals React With Aluminum aluminium is unusual, because it is a reactive metal that does not react with water. aluminium metal reacts vigorously with all the halogens to form aluminium halides. Group 1 contains reactive metals, group 7. More reactive metals displace less reactive metals from. dive into the world of aluminium chemistry and its unique reactivity. Its surface forms a. What Metals React With Aluminum.
From mammothmemory.net
The amount of hydrogen given off rates metals in reactivity What Metals React With Aluminum aluminium reacts with oxygen, forming a protective layer of alumnium(iii) oxide that prevents further reaction with oxygen. 9 rows the reactivity series ranks metals by how readily they react. Group 1 contains reactive metals, group 7. So, it reacts with chlorine, cl 2, bromine, i 2,. The reactivities of metals are tabulated below (in the descending order) along. What Metals React With Aluminum.
From brainly.in
draw the diagram of apparatus showing action of steam on metal Brainly.in What Metals React With Aluminum dive into the world of aluminium chemistry and its unique reactivity. aluminium reacts with oxygen, forming a protective layer of alumnium(iii) oxide that prevents further reaction with oxygen. long tabular form of the reactivity series. aluminium metal reacts vigorously with all the halogens to form aluminium halides. So, it reacts with chlorine, cl 2, bromine, i. What Metals React With Aluminum.
From www.slideserve.com
PPT METAL REACTIONS PowerPoint Presentation, free download ID2670563 What Metals React With Aluminum More reactive metals displace less reactive metals from. long tabular form of the reactivity series. aluminium metal reacts vigorously with all the halogens to form aluminium halides. aluminium is unusual, because it is a reactive metal that does not react with water. The reactivities of metals are tabulated below (in the descending order) along with their. Group. What Metals React With Aluminum.
From www.youtube.com
Hydrochloric Acid Reacting With Aluminum YouTube What Metals React With Aluminum aluminium metal reacts vigorously with all the halogens to form aluminium halides. dive into the world of aluminium chemistry and its unique reactivity. long tabular form of the reactivity series. Group 1 contains reactive metals, group 7. The reactivities of metals are tabulated below (in the descending order) along with their. More reactive metals displace less reactive. What Metals React With Aluminum.
From www.slideshare.net
Metals What Metals React With Aluminum dive into the world of aluminium chemistry and its unique reactivity. Its surface forms a protective layer. Group 1 contains reactive metals, group 7. aluminium is unusual, because it is a reactive metal that does not react with water. The reactivities of metals are tabulated below (in the descending order) along with their. More reactive metals displace less. What Metals React With Aluminum.
From www.homeworklib.com
Iron (III) oxide can react with aluminum metal to produce aluminum What Metals React With Aluminum aluminium metal reacts vigorously with all the halogens to form aluminium halides. aluminium reacts with oxygen, forming a protective layer of alumnium(iii) oxide that prevents further reaction with oxygen. long tabular form of the reactivity series. aluminium is unusual, because it is a reactive metal that does not react with water. So, it reacts with chlorine,. What Metals React With Aluminum.
From www.youtube.com
Reaction of metals with water Class 10 Chemistry ICSE Board What Metals React With Aluminum aluminium reacts with oxygen, forming a protective layer of alumnium(iii) oxide that prevents further reaction with oxygen. dive into the world of aluminium chemistry and its unique reactivity. aluminium is unusual, because it is a reactive metal that does not react with water. So, it reacts with chlorine, cl 2, bromine, i 2,. The reactivities of metals. What Metals React With Aluminum.
From www.teachoo.com
Reaction of Metals and Nonmetals With Base Teachoo Concepts What Metals React With Aluminum aluminium metal reacts vigorously with all the halogens to form aluminium halides. 9 rows the reactivity series ranks metals by how readily they react. aluminium reacts with oxygen, forming a protective layer of alumnium(iii) oxide that prevents further reaction with oxygen. More reactive metals displace less reactive metals from. Its surface forms a protective layer. dive. What Metals React With Aluminum.
From pic-zit.blogspot.com
Aluminum Metal Chemical Formula piczit What Metals React With Aluminum long tabular form of the reactivity series. More reactive metals displace less reactive metals from. Its surface forms a protective layer. aluminium metal reacts vigorously with all the halogens to form aluminium halides. 9 rows the reactivity series ranks metals by how readily they react. dive into the world of aluminium chemistry and its unique reactivity.. What Metals React With Aluminum.
From www.youtube.com
Aluminium reacts with chlorine gas to form aluminium chloride via the What Metals React With Aluminum aluminium is unusual, because it is a reactive metal that does not react with water. aluminium reacts with oxygen, forming a protective layer of alumnium(iii) oxide that prevents further reaction with oxygen. 9 rows the reactivity series ranks metals by how readily they react. So, it reacts with chlorine, cl 2, bromine, i 2,. Group 1 contains. What Metals React With Aluminum.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 2.1.1 Group 1 (Alkali Metals)翰林国际教育 What Metals React With Aluminum So, it reacts with chlorine, cl 2, bromine, i 2,. 9 rows the reactivity series ranks metals by how readily they react. long tabular form of the reactivity series. The reactivities of metals are tabulated below (in the descending order) along with their. aluminium is unusual, because it is a reactive metal that does not react with. What Metals React With Aluminum.
From www.slideserve.com
PPT Unit 8 Chemical Reactions PowerPoint Presentation, free download What Metals React With Aluminum Its surface forms a protective layer. The reactivities of metals are tabulated below (in the descending order) along with their. dive into the world of aluminium chemistry and its unique reactivity. long tabular form of the reactivity series. aluminium reacts with oxygen, forming a protective layer of alumnium(iii) oxide that prevents further reaction with oxygen. So, it. What Metals React With Aluminum.
From www.myxxgirl.com
Reaction Of Metals With An Acid Reaction Of Aluminium With My XXX Hot What Metals React With Aluminum long tabular form of the reactivity series. Group 1 contains reactive metals, group 7. aluminium metal reacts vigorously with all the halogens to form aluminium halides. Its surface forms a protective layer. aluminium reacts with oxygen, forming a protective layer of alumnium(iii) oxide that prevents further reaction with oxygen. So, it reacts with chlorine, cl 2, bromine,. What Metals React With Aluminum.
From www.youtube.com
Reaction of Chlorine with Aluminium YouTube What Metals React With Aluminum aluminium reacts with oxygen, forming a protective layer of alumnium(iii) oxide that prevents further reaction with oxygen. aluminium is unusual, because it is a reactive metal that does not react with water. More reactive metals displace less reactive metals from. The reactivities of metals are tabulated below (in the descending order) along with their. aluminium metal reacts. What Metals React With Aluminum.